This publication on stroke prevention and management highlights the need to reduce the local burden of stroke through the screening of individuals for stroke risk management and through ensuring a prompt and effective treatment response after stroke onset.
Reducing the burden of stroke
Stroke is a leading cause of death worldwide, with an estimated 5.7 million deaths from stroke in 2005, more than 1.4 million of these in China [1]. In Hong Kong, the annual stroke mortality rates are in excess of 3000 [2]. Stroke is also the commonest cause of permanent disability in adults [2]. The death and disability from stroke is expected to increase in Hong Kong as the population rapidly ages in coming decades [2].
Reducing the burden of stroke in Hong Kong, as elsewhere, depends on effective intervention, specifically:
• Promoting public awareness of stroke through stroke screening & public education
• Screening, assessment and intervention for modifiable risk factors for stroke;
• Consistent use of effective medical treatments for primary and secondary prevention of stroke;
• Prophylactic surgical or endovascular treatments for any organic vascular lesions found during stroke screening
• Rapid recognition of stroke in the community; and
• Timely and effective acute stroke management.
These topics, particularly medical and surgical aspects of stroke prevention and acute treatment, are the focus of this article. However, it is recognised that stroke and stroke recovery has a wider impact on families, carers and the community and that multidisciplinary rehabilitation is a key component of care for stroke survivors.
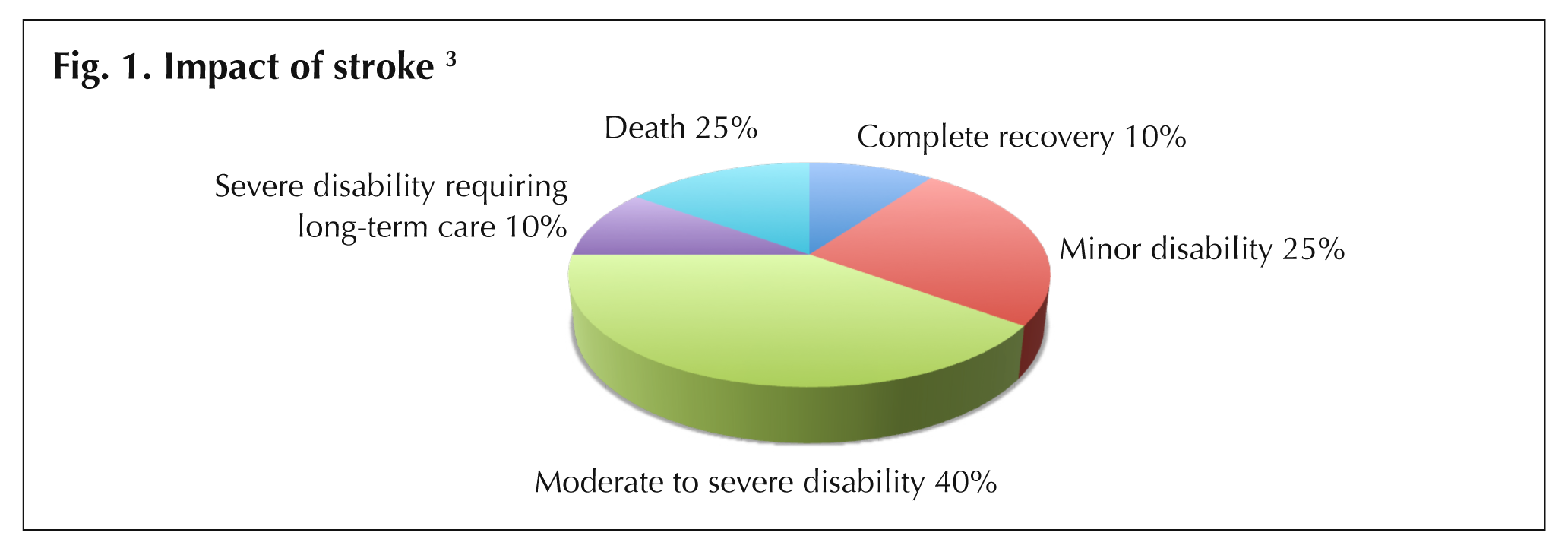
Stroke survivors experience a variety of outcomes that are determined by their age, severity of stroke, and the success and timing of treatment [3]. General stroke recovery rates are shown in Fig. 1 (Impact of stroke).
Improved treatments and more timely treatment have the potential to positively influence these outcomes for both individuals and their families.
 Risk factors for strokeScreening for stroke
Risk factors for strokeScreening for stroke
The majority of strokes are preventable and thus prevention continues to be a key aspect of reducing the burden of stroke for individuals and the wider community [4]. This can be achieved through screening individuals for known risk factors for stroke and implementing management of modifiable risk factors. In the United States, most stroke centres recommend starting stroke screening for adults from 18 years of age.
Recognised risk factors for ischaemic stroke are summarised in Table 1, with modifiable risk factors signifying potential areas for lifestyle, medical or surgical interventions.
| Table 1. Risk Factor for ischaemic stroke [5,6] | |
| Modifiable risk factors | |
| • Obesity | • Other extra or intracranial vascular abnormalities |
| • Smoking | • Coronary heart disease |
| • Alcohol abuse | • History of stroke or TIA |
| • Lack of exercise | • Atrial fibrillation |
| • Hypertension | • Valvular heart diseases |
| • Diabetes mellitus | • Hypercoagulable states |
| • Hypercholesterolaemia | • Homocysteinaemia |
| • Severe carotid stenosis | • Connective tissue diseases |
| • Intracranial arterial stenosis | |
| Not able to be modified | |
| • Increasing age | • Family history |
| • Male sex | • Race / ethnic group |
Insights from the INTERSTROKE study
The recently published findings of the INTERSTROKE study [6] have added a great deal to the understanding of specific risk factors for stroke. This study, conducted in 22 countries worldwide, investigated risk factors for ischaemic and intracerebral haemorrhagic stroke in 3000 cases with 3000 matched controls.
The INTERSTROKE study found that the 10 most significant risk factors for all stroke were: hypertension, current smoking, waist-to-hip ratio, diet risk score, low levels of physical activity, diabetes mellitus, alcohol intake of more than 30 drinks per month or binge drinking, psychosocial stress, depression, and cardiac causes. These risk factors collectively accounted for approximately 90% of the risk of stroke.
In keeping with earlier studies, the INTERSTROKE study found that hypertension is the most important risk factor for all types of stroke and is a more potent risk factor for haemorrhagic than ischaemic strokes. Hypertension was also shown to be a particularly important risk factor in people 45 years or younger.
Screening at the hong kong stroke centre
Screening at the hong kong stroke centre is a comprehensive process. It includes:
• History taking and interviewing patients about their lifestyle, personal health, family history and stroke risk
• Clinical and laboratory screening to assess for risk factors
• Imaging screening of the brain using magnetic resonance imaging, and intracerebral and cervical vessel imaging using magnetic resonance angiography
• Risk factor control interventions appropriate to each individual
- Lifestyle modification (eg, dietary advice & weight management, smoking cessation advice, etc)
- Medical therapy (for blood pressure, cholesterolaemia, glycaemic control in diabetes etc)
• Neurosurgical endovascular management (eg, intracranial or extracranial vascular abnormalities, carotid artery stenosis, etc)
Screening promotes stroke prevention and early intervention at the level of each individual patient. It takes a proactive approach to the identification of risks for stroke, including the identification of vascular abnormalities, stenosis, and existing lesions using diagnostic imaging, and also provides interventions as required. Comprehensive stroke screening at the hong kong stroke centre assists the family physician in the ongoing management of each patient.
 |
|
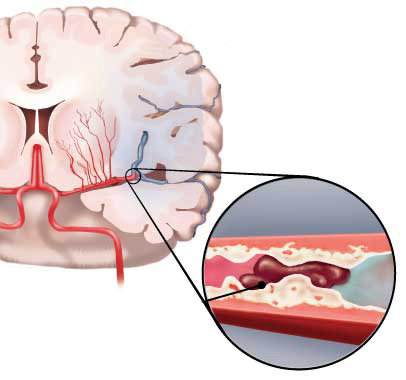
| Fig. 2. Ischaemic stroke: blood clot stops the flow of blood to an area of the brain |
Stroke: New concepts and care
Most strokes are acute ischaemic strokes due to blockage of an artery in the brain (Fig. 2). In Hong Kong, it is estimated that approximately 70% of strokes are ischaemic strokes [5], with the remainder being haemorrhagic strokes. Stroke is a heterogeneous condition and the treatment strategy for these two stroke types differs. It is important that the care of each patient at each phase of intervention is tailored to the individual's needs [5].
The major development in stroke care in the last decade is the recognition that acute ischaemic stroke is a medical emergency and that 'time is brain' (see box feature) [7]. This has coincided with the availability of thrombolytic treatment for ischaemic stroke using intravenous recombinant tissue plasminogen activators (rtPAs). More recently, advances have included Percutaneous Cerebral Intervention (Neuro-PCI). These treatments are available at the hong kong stroke centre, with the aim of minimising stroke disability and improving outcomes for individuals with acute ischaemic stroke.
|
Time is Brain • Emergency evaluation and treatment of acute ischaemic stroke is required. Estimates of brain loss per unit time highlight the time urgency of stroke care. • The most common type of ischaemic strokes are large vessel, supratentorial strokes. These strokes typically have an evolution of between 6 to 18 hours. During stroke evolution, it is estimated that 120 million neurons, 830 billion synapses and 714 km of myelinated fibres are lost each hour (Table 2). • Compared with the normal rate of neuronal loss in aging, the ischaemic brain ages 3.6 years each hour of stroke evolution without treatment.
source: Saver JL. Stroke.2006;37:263-266 |
||||||||||||||||||||||||||||
Community stroke recognition and response
A critical step in improving stroke outcomes is ensuring that there is prompt recognition of stroke and rapid access to appropriate treatment facilities. This is essential to ensure patients receive treatment within the therapeutic window for appropriate treatments [8].
Delays in seeking medical attention after stroke/TIA reflect that the patient and those with them fail to recognise the symptoms of stroke/TIA or to seek emergency medical care [9]. The need for greater public awareness is recognised, particularly for those at increased risk of stroke. Through stroke screening, public awareness can be promoted.
The FAST mnemonic (face, arm speech, time) has been shown to identify almost 90% of cases of acute stroke and TIA, with better performance for ischaemic than haemorrhagic stroke [9]. The FAST mnemonic has been used in public awareness campaigns around the world. See the box feature for further details of the FAST.
|
Recognise stroke symptoms F.A.S.T. You can recognise a stroke using the FAST test FACIAL weakness: Can the person smile? Has their mouth or eye drooped? ARM weakness: Can the person raise both arms? SPEECH problems: Can the person speak clearly and understand what you say? TIME to call for emergency medical help: 24-hour emergency hotline +852 3420 6660 If a person fails any one of these tests, get help immediately by dialling 999 A speedy response can help reduce the damage to a person's brain and improve their chances of a full recovery. A delay in getting help can result in death or long-term disabilities. Don't ignore temporary symptoms If symptoms disappear within 24 hours, the person may have had a transient ischaemic attack (TIA), which is also called a mini-stroke. A TIA is still a medical emergency, because it can lead to a major stroke. This version of the FAST has been adapted for asia medical specialists from a version of the FAST content available at www.shetlandlive.com |
3 Golden hours: Benefits and limitations of intravenous rtPA
Intravenous rtPA is a proven, established treatment for acute ischaemic stroke [10,11] and is recommended in local and international guidelines for acute stroke treatment [4,5,12]. It needs to be delivered in a well-equipped centre with relevant expertise to allow rapid assessment, investigation, treatment & monitoring [12]. Accepted indications for use of this treatment are as presented in Table 3.
The therapeutic benefit of IV rtPA is strongly time dependent and is greatest when given early after stroke onset and declines over 3 to 4.5 hours. Studies have shown that starting this treatment within 90 minutes of stroke onset is optimum and that for every 10 minute delay within the 1-3 hour treatment time period, 1 fewer patient has an improved disability outcome [10]. This has led to initiatives aimed at achieving thrombolysis - so-called 'door to needle' times - of within 60 minutes. The American Heart Association/American Stroke Association is currently promoting a goal of 50% of patients with ischaemic stroke having a door to needle time of ≤60 minutes [13].
Key strategies identified to meet this goal include:
• Emergency medical service pre-notification
• Activating the stroke team with a single call
• Rapid access to and interpretation of brain imaging
• Specific protocols and tools for acute stroke management
• A team-based approach
Symptomatic intracranial bleeding is a recognised and serious side effect of IV rtPA and is estimated to affect approximately 6% of patients [11]. Salvage neurosurgery is a difficult undertaking if intracranial bleeding occurs but is an option in specialist neurosurgical centres, such as the hong kong stroke centre.
| Table 3. Patient Selection Criteria for IV rtPA |
| Indications [12] |
| • Onset of ischaemic stroke within the preceding 3 hours • Measurable and clinically significant deficit on National Institute of Health Stroke Scale (NIHSS) examination • Patient's brain imaging does not show haemorrhage or non-vascular cause of stroke • Patient's age is >18 years |
| Contraindications [14] |
| • Age < 18 • CT scan findings (intracranial haemorrhage, or major acute infarct signs) • Suspicion of subarachnoid haemorrhage (even if head CT is negative for haemorrhage) • Recent (within 3 months) major surgery or trauma (discuss with Attending) • History of intracranial haemorrhage or brain aneurysm or vascular malformation or brain tumor (May consider IV tPA in patients with CNS lesions that have a very low likelihood of haemorrhage, such as small unruptured aneurysms or benign tumors with low vascularity) • Known bleeding diathesis OR 1. Current use of oral anticoagulants with INR > 1.7 or PT > 15 seconds 2. Use of heparin within 48 hours preceding onset of stroke AND prolonged aPTT at time presentation 3. Platelets <100,000 4. Internal haemorrhage (GI haemorrhage, urinary tract haemorrhage) < 3 weeks 5. Dabigatran use in the past 48 hours (if last dose >48 hours, confirm normal renal function [creatinine clearance >50 mL/min] and normal coagulation [aPTT, INR, platelet count] before tPA administration). 6. Low molecular weight heparin use (i.e.- Lovenox) in the past 24 hours. • Persistent systolic BP >185 mm Hg or diastolic BP >110 mm Hg despite treatment. |
8 Golden hours: Neurointerventional techniques for acute ischaemic stroke
Neuro-PCI
• Intra-arterial thrombolysis (ia rtPA)
• Mechanical thrombectomy
• Intravascular stenting
These treatments can be offered in highly specialised centres with advanced imaging and neurosurgical specialisation. They may be used alone or in combination. A major advantage of these treatments is that they are of benefit beyond the period that intravenous rtPA thrombolysis is effective. They extend the therapeutic window for acute ischaemic stroke treatment from 3 hours, offering up to '8 Golden hours'.
Neuro-PCI treatment depends on detailed brain angiography to look for the causative vascular occlusive lesion that PCI will target. The use of neuro-PCI for stroke is similar to the use of cardiac PCI for acute coronary ischaemia. In the United States, comprehensive stroke centres with a vascular neurosurgical service may prefer neuro-PCI over intravenous rtPA treatment because of the reduced risk of bleeding complications. When intra-arterial rtPA is used as part of a neuro-PCI intervention, a very low dose of rtPA is directly infused as an adjunct to mechanical thrombectomy to lyse the blocking thrombus.
Recent data from the MERCI/Multi MERCI trials patients showed that as many as 40% of those who had successful revascularisation with such treatments at 6.9 hours or later after stroke onset still achieved independent functional outcomes [15].
Appropriate treatment should be started as soon as possible after stroke onset for the best outcome. However, in special clinical conditions some patients presenting up to 8 hours after stroke onset, can still benefit from neurointervention.
Mechanical thrombectomy
Brain revascularisation can be achieved by mechanical thrombectomy under the fluoroscopic guidance of cerebral angiograms (Fig. 4). There are a range of clot retrieval devices that can potentially be used for mechanical thrombectomy [16].
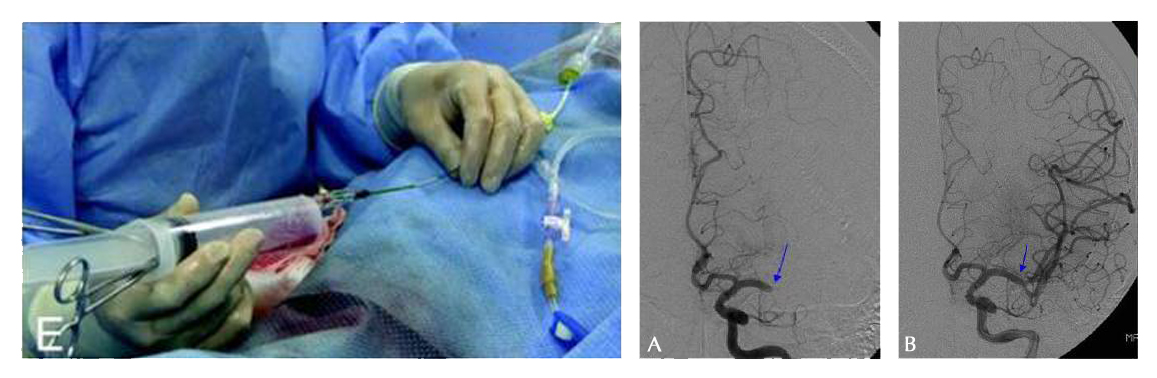 |
| Fig. 4. Mechanical thrombectomy revascularising the brain A - Pretreatment angiography shows total occlusion of the M1 segment of the left middle cerebral artery (MCA) (arrow). B - Immediate post-procedural angiography shows complete revascularisation (arrow). |
With clot retrieval devices, a guidewire and micro-catheter is passed up to the occluding clot in the intracranial vessel. The thrombus or embolus is then removed by the device using mechanical breakage and aspiration. Clot retrieval devices, such as the MERCI Retrieval System (Concentric Medical Inc., California, USA) (Fig. 5) have been the most well-researched [16] and the MERCI system has now been in use in the USA and Europe for several years [17]. A systematic review of 23 small observational studies found that those treated with clot removal devices were about 15 times more likely to have a good outcome (modified Rankin score ≤2) and 2.2 times less likely to die [16].
The Penumbra System (Penumbra, Alameda, California, USA) [17] is a more recent device also designed for use in acute ischaemic stroke secondary to large vessel thromboembolism (Fig. 6).
 |
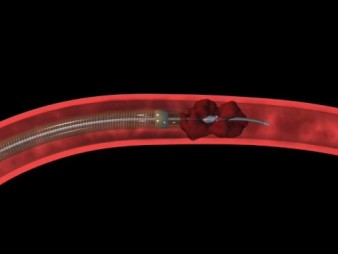 |
| Fig. 5. The Merci Clot retriever for mechanical thrombectomy | Fig. 6. The Penumbra System for mechanical thrombectomy |
Intra-arterial thrombolysis
Injection of IA rtPA at the site of the thromboembolism improves outcomes in acute ischaemic stroke. A Cochrane review found that IA thrombolysis resulted in a significant reduction in the combined odds of death and dependency at follow-up [11].
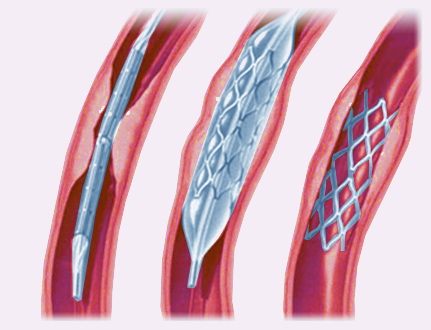
Use of intravascular stents
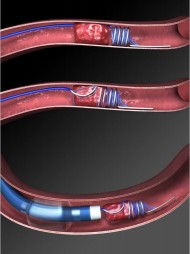
With advances in endovascular stent design and delivery, stenting has recently become a feasible treatment option for neurovascular disease [18]. Stents initially conceived for treatment of intracranial atherosclerosis (Wingspan stent - Fig. 7) and for aneurysm remodelling (Enterprise and Neuroform stents) have paved the way for new therapeutic approaches in acute ischaemic stroke [19].
 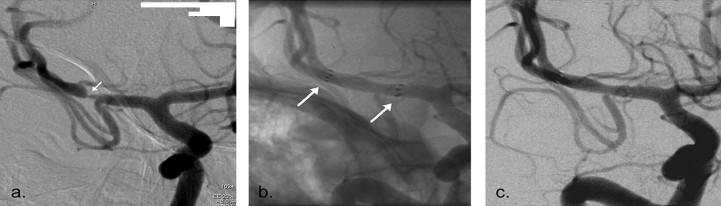 |
| Fig. 7. The Wingspan stent. A - The Pre-stenting angiogram (DSA) showing right MCA stenosis B - stent-in-situ with stent marker (white arrow). C - the post-stenting DSA |
Clinical studies to date have shown that stents are efficacious in the treatment of acute ischaemic stroke [18].
| Potential advantages of neuro-PCI | |
| Neuro-PCI | Intravenous rtPA |
|
Extends the therapeutic window from 3 hours up to 8 hours. Neuro-PCI offers a treatment option for those with acute stroke who do not access specialist medical services within 3 hours. Effective neuro-PCI revascularisation in the 4th to 7th hour may salvage brain cells in the Penumbra Zone (peripheral ischaemic region) minimising the area of infarction. |
Accepted therapeutic window for treatment is 3 hours. Studies show optimum use is within 90 minutes [10]. These time-to-treatment intervals have proved generally difficult to achieve due to patient factors, lack of community awareness and access and provision of appropriate medical services. |
|
Neuro-PCI may be suitable for individual patients who: a) have a contraindication to intravenous rtPA; or b) have not had an adequate therapeutic response to intravenous rtPA. |
Symptomatic intracranial bleeding is a recognised and serious side effect of IV rtPA and is estimated to affect approximately 6% of patients [11]. |
| Intra-arterial delivery of lytic agents may increase the chance of recanalisation. | Occlusions in some areas (e.g. proximal middle cerebral artery) are known to be more resistant to intravenous delivery of lytic agents [20]. |
Summary
Stroke is a brain attack and time is brain.
Prevention through screening and risk factor control is the best possible treatment.
Awareness of stroke symptoms and rapid access to emergency medical care is necessary to improve outcomes.
Neurointervention techniques now allow effective acute ischaemic stroke treatment for up to a GOLDEN 8 HOURS.
References
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. The Lancet Neurology. 2007;6:182-7.
- Ng PW. The stroke epidemic. Hong Kong Med J 2007;13:92-4.
- National Stroke Association. Stroke facts. http://www.stroke.org Accessed 26 June 2012.
- Schwamm L, Fayad P, Acker JE III, Duncan P, Fonarow GC,Girgus M. Translating evidence into practice: a decade of efforts by the American Heart Association/American Stroke Association to reduce death and disability due to stroke: A Presidential advisory for the American Heart Association/American Stroke Association. Stroke 2010;41:1051-65
- Ng PW, Huang CY, Cheung RTF, Wong KS, Wong CK, Lam JK for the Hong Kong Neurological Society and Hong Kong Stroke Society. Consensus statement on ischaemic stroke care in Hong Kong. Hong Kong Med J 2004;10:124-9.
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al: INTERSTROKE investigators. Risk factors for ischaemic and intracerebral aemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;10:112-23.
- Saver JL. Time is brain – quantified. Stroke 2006;37:263-266.
- Kleindorfer DO, Miller R, Moomaw CJ, Alwell K, Broderick JP,Khoury J, et al. Designing a message for public education regarding stroke. Does FAST capture enough stroke? Stroke 2007;38:2864-2868.
- Sprigg N, Machilli C, Otter ME, Wilson A, Robinson TG. A systematic review of delays in seeking medical attention after transient ischaemic attack. J Neurol Neurosurg Psychiatry 2009;80:871-5.
- Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischaemic stroke. Circulation 2011;123:750-758.
- Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Reviews. 2009. CD000213.
- National Stroke Foundation (Australia). Clinical Guidelines for Stroke Management 2010. Melbourne, Australia. www.strokefoundation.com.au
- Fonarow GC, Smith EE, Saver JL, Reeves MJ, Hernandez AF, Peterson ED, et al. Improving door-to-needle times in acute ischaemic stroke. The design and rationale for the American Heart Association/American Stroke Associations's Target: Stroke initiative. Stroke 2011;42:2983-2989.
- Boston Medical Center. Acute Stroke Protocol. http://www.bmc.org/stroke-cerebrovascular/services/acute-stroke-protocol.htm Accessed 20 August 2012.
- Nogueira PC, Smith WS, Sung G, Duckwiler G, Walker G, Roberts R, et al. Effect of time to reperfusion on clinical outcome of anterior circulation strokes treated with thrombectomy. Stroke 2011;42:3144-3149.
- Stead LG, Gilmore RM, Bellolio MF, Rabinstein AA, Decker WW. Percutaneous clot removal devices in acute ischaemic stroke: A systematic review and meta-analysis. Arch Neurol 2008:65:1024-30.
- Bose A, Henkes H, Alfke K, Reith W, Mayer TE, Berlis A, Branca V, Po Sit S, for the Penumbra Phase I Stroke Trial Investigators. The Penumbra System: A mechanical device for the treatment of acute stroke due to thromboembolism. AJNR 2008;29:1409-1413.
- Ansari S, McConnell DJ, Velat GJ, Waters MF, Levy EI,Hoh BL, et al. Intracranial stents for treatment of acute ischaemic stroke: evolution and current status. World Neurosurg 2011;76:S24-34.
- Samaniego FA, Dabus G, Linfante I. Stenting in the treatment of acute ischemic stroke: literature review. Front Neurol 2011;2:76. Epub 2011 Dec 9.
- Meyers PM, Schumacher C, Sander Connolly E, Heyer EJ, Gray WA, Higashida RT. Current status of endovascular stroke treatment. Circulation 2011;123:2591-2601.